Rate coefficients for reactions of nitrate radicals (NO 3) with (Z)pent2ene, (E)pent2ene, (Z)hex2ene, (E)hex2ene, (Z)hex3ene, (E)hex3ene and (E)3methylpent2ene were determined to be (655 ± 078) × 10 −13 cm 3 molecule −1 s −1, (378 ± 045) × 10 −13 cm 3 molecule −1 s −1, (530 ± 073) × 10 −13 cm 3 molecule −1 s −1, (3 ± 047) × 10 −13 cm2bromo3methylpentane 0 7 1 The alkene 3methylpent2ene (CH 3CH=C(CH 3)CH 2CH 3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene 1 mark 0 7 2 Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide Explain why more 3bromoThe alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) reacts with hydrogen bromide to form a mixture of 3bromo3methylpentane and 2bromo3methylpentane (a) The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene 1 (b) Name and outline the mechanism for the formation of 3
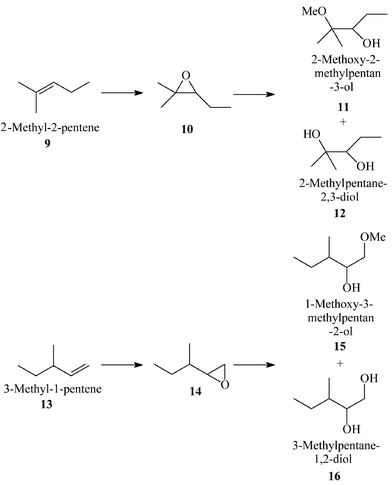
Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing Doi 10 1039 425g
E 3 methylpent 2 ene
E 3 methylpent 2 ene-File (E)3methylpent2ene 0svg Size of this PNG preview of this SVG file 162 × 56 pixels Other resolutions 3 × 111 pixels 640 × 221 pixels 800 × 277 pixels 1,024 × 354 pixels 1,280 × 442 pixels 2,560 × 5 pixelsInChI=1/C6H11Cl/c146 (7)5 (2)3/h45H,13H3/b64 Molecular Formula C6H11Cl Reactions where this compound is a product ( Cross Metathesis) 0 from prop1ene, 2chloro3methylbut1ene from 2chloro3methylbut1ene, prop1ene Reactions where this compound is



Scilearn Sydney Edu Au Fychemistry Textbook Chapter15 Pdf
trans2fluoro3methylpent2ene this compound in also known as (Z)2fluoro3methylpent2ene I have given my possible structure in the image but itA reaction of an unknown alkene with MCPBA in dichloromethane followed by workup with H2O/H yielded, as the major product, a racemic mixture of (2S, 3S) and (2R, 3R)3methylpentan2,3diol What is the specific structure of the alkene used in the reaction?Find SigmaAldrich MSDS, related peerreviewed papers, technical documents, similar products & more at SigmaAldrich
Start researching unis here >> start new discussion replyExplain why 3methylpent2ene does not display cis trans isomerism, but does display E/Z isomerism c Does 2methylpent2ene satisfy any of the criteria?4 Briefly explain how to assign priority to groups using CIP nomenclature a Use CHClC(CH 3)CH 2 CH 3 to demonstrate
3methylpent2ene isomer Watch Announcements Aged 1418?Chemistry questions and answers Question 13 (05 points) Name the following compound O a) (R,E)2,4dibromo3methylpent3ene Ob) (S,E)2,4dibromo3methylpent3ene Oc) (R,Z)2,4dibromo3methylpent2ene d) (S,E)2,4dibromo3methylpent2ene Oe) (RE)2,4dibromo3methylpent2ene Question 14 (05 points) What is the correct IUPAC nameFind 3methylpent2ene and related products for scientific research at MilliporeSigma



Chemsheets As006 Electron Arrangement Ppt Download



1
C6H12, (E)3Methyl2pentene, Molecules Containing Five or More Carbon Atoms DOI /_447 Study of the composition of gas from the catalytic cracking of vacuum gas oil, by means of capillary gas chromatography, Chemistry and Technology of Fuels and OilsMethylpent2ene C Z3ethylbut2ene D ethylbut2ene A What is the effect produced by the PRK technique designed to correct nearsightedness?3Methylpent2ene (CH3CH=C(CH3)CH2CH3) reacts with Hydrogen Chloride(HCl) forming a major and minor product Please name the reaction, draw the mechanism for the formation of the major product and briefly explain why there is a major and a minor product



Which Of The Following Compounds Are Capable Of Ciis And Trans Isomerism Why 4 Methylpent 2 Ene 3 Methylpent 1 Ene 4 Chlorohex 2 Ene Quora
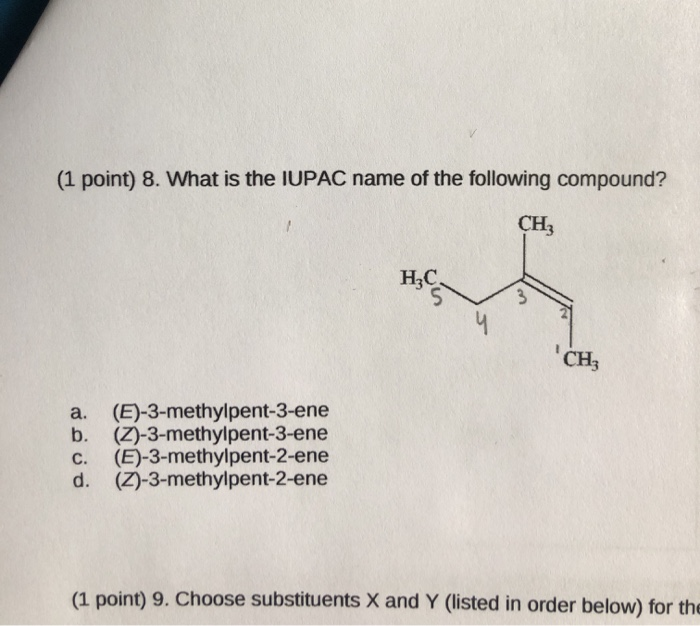



1 Point 8 What Is The Iupac Name Of The Following Chegg Com
Find more compounds similar to (E)3Ethyl4methylpent2ene Note Cheméo is only indexing the data, follow the source links to retrieve the latest data The source is also providing more information like the publication year, authors and more Take the time to validate and double check the source of the data(E)3Methylpent2en Chemische Eigenschaften,Einsatz,Produktion Methoden RSätze Betriebsanweisung R11Leichtentzündlich R65Gesundheitsschädlich kann beim Verschlucken Lungenschäden verursachen SSätze Betriebsanweisung S9Behälter an einem gut gelüfteten Ort aufbewahren S16Von Zündquellen fernhalten Nicht rauchen› cis 4 methylpent 2 ene Filter by All Education Study Learning Search 4Methyl1pentene C6H12 PubChem › See more all of the best education on wwwnihgov Education Laboratory Chemical Safety Summary (LCSS) Datasheet Molecular Formula C6H12 Synonyms 4METHYL1PENTENE




Z 3 Ethyl 4 Methylpent 2 Ene Structure C8h16 Over 100 Million Chemical Compounds Mol Instincts



2 Methylpent 2 Ene Get Quote
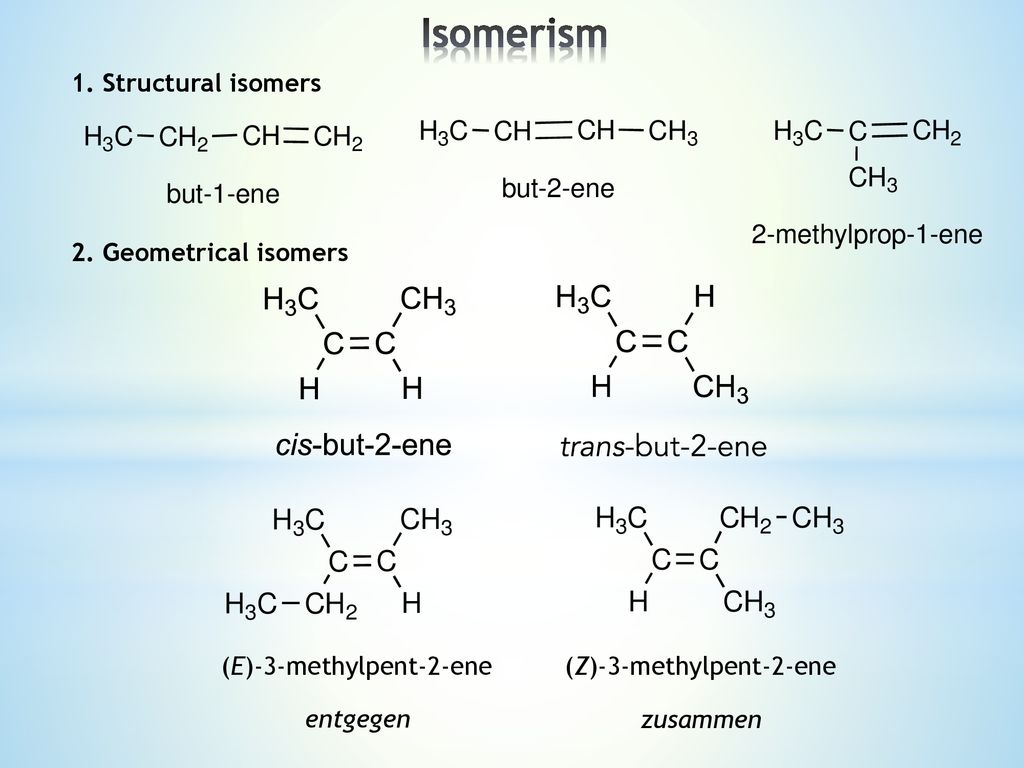
A) (Z)3methylpent2ene B) (E)3methylpent 2ene C) 2methylpent2ene Now there are two possible isomers, because the = is inflexible The Zisomer where the stuff left of the = is at the same side of the C = C as the stuff on the right, so both down or both up (from German Zusammen=together) The Eisomer where they are on opposite sides (from Entgegen=opposite) Note They are also called cis (Z) and trans (E)A The density of the cornea is increased B The radius of curvature of the cornea is increased C
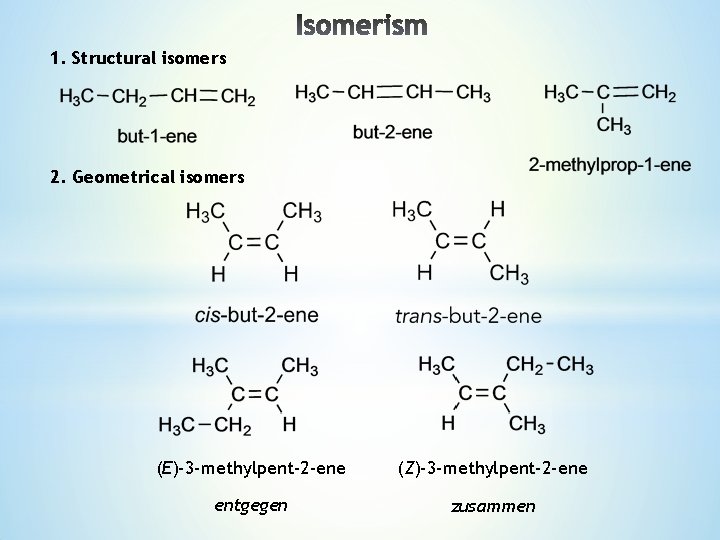



Lecture 4 1 Structural Isomers 2 Geometrical Isomers



How Many Different Alkenes Can Be Hydrogenated To Form 3 Methylpentane Socratic
The (E)1chloro3methylpent2ene molecule consists of 11 Hydrogen atom(s), 6 Carbon atom(s) and 1 Chlorine atom(s) a total of 18 atom(s) The molecular weight of (E)1chloro3methylpent2ene is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be Chemsrc provides 2Pentene, 3methyl,(2Z)(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of 2Apply to join TSR's Student Advisory Board have your voice heard, get wicked experience for your CV and help support students Applying to uni in 22?




E 2 Cyanoperfluoro 3 Methylpent 2 Ene Spectrabase




13 0 Alkenes Exam Q S Flashcards Quizlet
The compound produced when 3methylpent2ene undergoes hydrogenation in the presence of a platinum catalyst is _____ 3methylpentane 9 Using Zaitsev's rule, choose the most stable alkene among the following A) 1methylcyclohexene B) 3methylcyclohexene C) 4methylcyclohexeneNIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 Professional Edition Version 2121Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics These data were generated through dynamic data analysis, as implemented in the NISTA)methypent2ene Stepbystep explanation In z mechanism ,the compounds with higher priority will be located opposite to each other of the double bond ,in E mechanism the compounds with high priority will be located in z corners and hence 3methylpent 2ene is one which show EZ mechanism in which the priority group in CH3 and CH2CH3
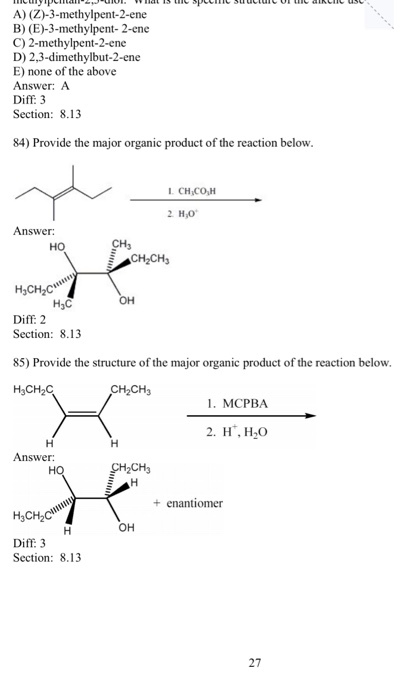



A Z 3 Methylpent 2 Ene B E 3 Methylpent 2 Ene Chegg Com
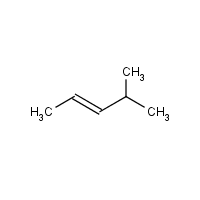



E 4 Methylpent 2 Ene Hazardous Agents Haz Map
E is a British owned energy supplier based in Birmingham We ensure everything is simple and straightforward for our customers Our focus is on keeping costs down so you pay less E is all about saving money Switch to us We ensure everything isBioaccumulation Estimates from Log Kow (BCFWIN v217) Log BCF from regressionbased method = 1709 (BCF = 5123) log Kow used 313 (estimated) Volatilization from Water Henry LC 05 atmm3/mole (estimated by Bond SAR Method) HalfLife from Model River hours (5623 min) HalfLife from Model Lake 8715 hours (3631 days) Removal InH3CCHCH CH 2 CH2 CH3 hex2ene (newer rules) 2hexene (older rules) 1 2 34 56 3 Indicate the position of any substituent group by the number of the carbon atom in the parent (longest) chain to which it is attached H3CCHCHCHCH2 CH CH3 CH3 CH3 5,6dimethylhept3ene (newer rules) 5,6dimethyl3heptene (older rules) 1 2 34 5 6 7



Z 3 Ethyl 4 Methylpent 2 Ene C8h16 Chemspider




Trans 3 Methyl 2 Pentene 99 0 Tci America Fisher Scientific
Chemical structure of (2E)3methylpent2ene See it's properties and synonymsOption second is correct The methylpent2ene E is for trans configuration Accordi View the full answer Transcribed image text Select the IUPAC name for the compound shown below 0 (E)2methylpent3ene (E)3methylpent2ene (E)3methylpent3ene O (Z)3methylpent2ene Previous question Next questionEnglish Structure of (Z)3methylpent2ene Deutsch Struktur von (Z)3Methyl2penten Date 12 March 14 Source Own work Author Emeldir SVG development The source code of this SVG is valid This structural formula was created with Name2Struct CS ChemDraw Ultra



E 3 Isopropyl 2 Methyl Pent 2 Ene 1 5 Diol C9h18o2 Chemspider



1
Gasphase rate coefficients for the reactions of nitrate radicals with (Z)pent2ene, (E)pent2ene, (Z)hex2ene, (E)hex2ene, (Z)hex3ene, (E)hex3ene and(a)€€€€ The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) 4 (b)€€€€ Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide(a) The alkene 3methylpent2ene (CH 3 CH=C(CH 3)CH 2 CH 3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) (b) Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide Explain why more 3bromo3methylpentane is formed in this



E 3 Methylpent 2 Ene Molbase



E 4 Chloro 3 Methyl Pent 2 Ene Chemsink
(2R,3S)2chloro3methylpentane reacts with sodium methoxide in methanol to give (Z)3 methylpent2ene as the major product and (E)3methylpent2ene as the minor product In the space below a) Write the structures of both products b) Write aHexene is an alkene with a molecular formula C 6 H 12The prefix "hex" is derived from the fact that there are 6 carbon atoms in the molecule, while the "ene" suffix denotes that there is an alkene present—two carbon atoms are connected via a double bondThere are several isomers of hexene, depending on the position and geometry of the double bond in the chain3 − M e t h y l − p e n t − 2 − e n e on reaction with H B r in presence of p e r o x i d e forms an addition product A molecule of H B r is added to C = C double bond The addition follows antiMarkowikoff's rule



E 3 Chloro 4 Methyl Pent 2 Ene Chemsink
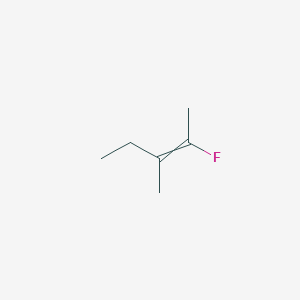



2 Fluoro 3 Methylpent 2 Ene C6h11f Pubchem
The compound is (E)but2ene A minor addition to the rule to allow for isotopes of, for example, hydrogen Deuterium is an isotope of hydrogen having a relative atomic mass of 2 It still has only 1 proton, and so still has an atomic number of 1 However, it isn't the same as an atom of "ordinary" hydrogen, and so these two compounds areLower right example methylpent2ene and Z3methylpent2ene (repeated further down with skeletal formulae) To understand the two lower left and right examples apply the Priority Rules to alkenes for E/Z ('geometrical') isomerism For each carbon of the double bond the higher priority atom/group is worked outAlso know as (E)2chloro3methylpent2ene, (E)2chloro3methylpent2ene, (E)2chloro3methylpent2ene, (E)2chloro3



E 3 Methylpent 2 Enenitrile Chemsink




Organic Chemistry Alkenes
Q select the incorrect statement answer choices In alkenes, the carbons are connected by pi bonds Alkenes have almost same physical properties as that of the alkanes Alkenes are less reactive than alkanes Alkenes undergo polymerization reactions s Question 3 Chemsrc provides 1chloro3methylpent2ene(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of 1chloro3methylpent2ene are included as well



2e 3 Methyl 2 Pentene C6h12 Chemspider




Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing Doi 10 1039 425g
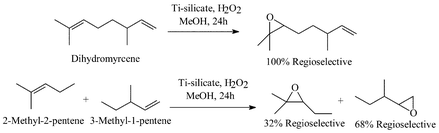



Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing




3 Methyl 3 Penten 2 One Wikipedia



Answer In Organic Chemistry For Edem



What Is The Molecular And Structural Formula Of 3 Methylpent 2 Ene Quora




Which Of The Following Is The Major Product In The Electrophilic Addition Of Hcl To 2 Methylpent 2 Ene Homeworklib




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Pero Innovayz



4461 48 7 4 Methylpent 2 Ene Cas No 4461 48 7 4 Methylpent 2 Ene



2747 48 0 2e 3 Methylpent 2 En 1 Ol Cas No 2747 48 0 2e 3 Methylpent 2 En 1 Ol
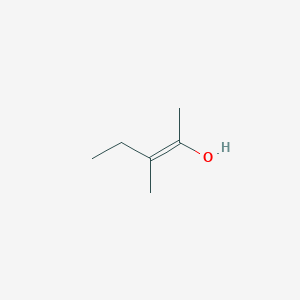



E 3 Methylpent 2 En 2 Ol C6h12o Pubchem




File E 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
-2-bromo-3-methylbutane_small.png)



Organic Chemistry Alkenes
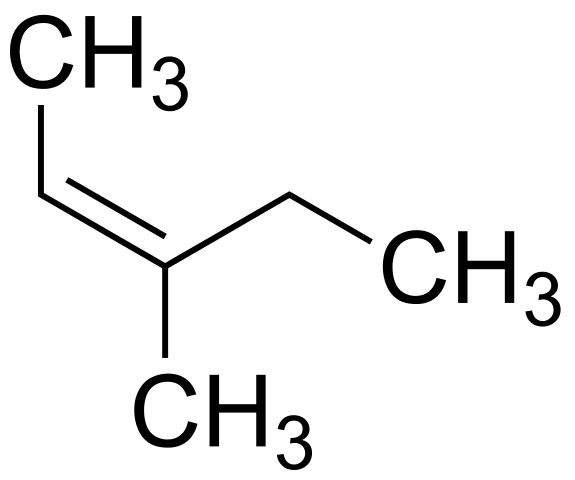



File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
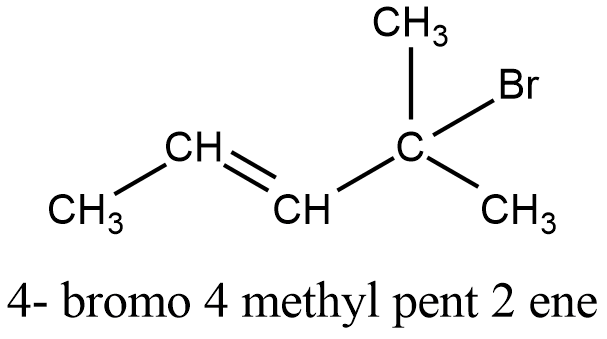



Write The Structure Of Four Bromo 4 Methyl Pent 2 Ene Chemistry Topperlearning Com N3bifjj



Scilearn Sydney Edu Au Fychemistry Textbook Chapter15 Pdf
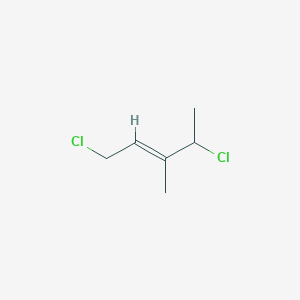



E 1 4 Dichloro 3 Methylpent 2 Ene C6h10cl2 Pubchem




S E 4 Hydroxy 3 Methyl Pent 2 Ene Nitrile Spectrabase




When 3 Methylpent 2 Ene Is Treated With Mercury Ii Acetate In Methanol And The Resulting Product Brainly Com



Z 3 Methylpent 2 Ene Chemsink
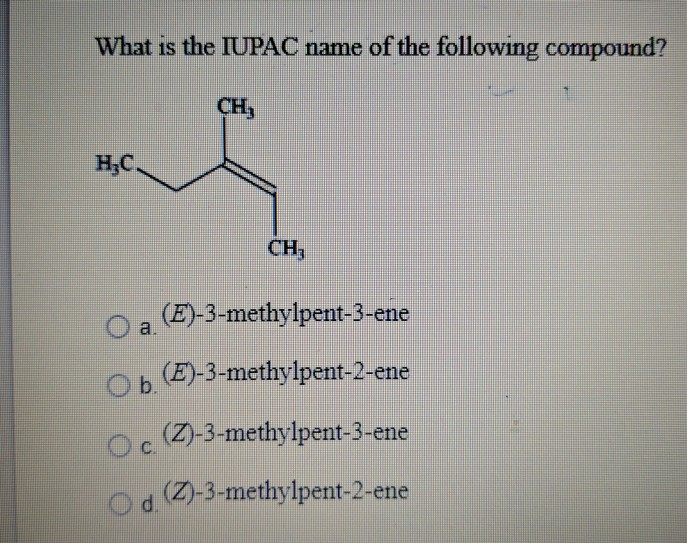



What Is The Iupac Name Of The Following Compound Ch Chegg Com



E 3 Methylpent 2 Ene Molbase



Z 3 Ethyl 4 Methylpent 2 Ene




Geometrical Isomerism Cis Trans In Trans 2 Fluoro 3 Methylpent 2 Ene Chemistry Stack Exchange



3 Methylpent 2 Ene 1 5 Diol C6h12o2 Chemspider



3 Ethyl 2 Methylpent 2 Ene Get Quote




File E 3 Methylpent 2 Ene 0 Svg Wikimedia Commons




Rank The Alkenes Below From Least To Most Stable A 2 3 Dimethylpent 2 Ene B 3 Ethylpent 2 Ene C 2 Methylhex 1 Ene D Cis Hept 2 Ene E Trans Hept 2 Ene F Hept 1 Ene Study Com




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons



Cid C6h11 Pubchem



Z 3 Ethyl 4 Methyl Pent 2 Ene Chemsink



1




How To Draw The Structure For 3 Methylpent 1 Ene Drawing Alkenes Organic Chemistry Youtube



2e 2 Bromo 5 Chloro 3 Methylpent 2 Ene



2e 4 Methylpent 2 Ene Get Quote
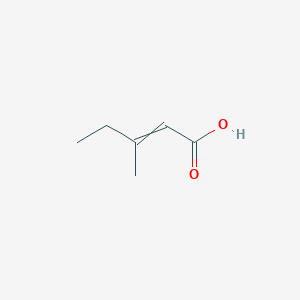



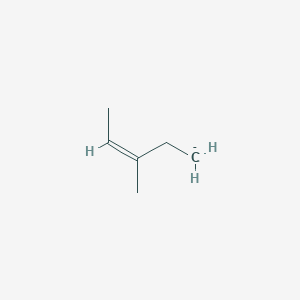
3 Methylpent 2 Enoic Acid C6h10o2 Pubchem



2z 3 Methylpent 2 Ene Get Quote




13 0 Alkenes Exam Q S Flashcards Quizlet
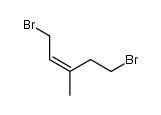



Z 1 5 Dibromo 3 Methylpent 2 Ene Cas 67 2 Chemsrc
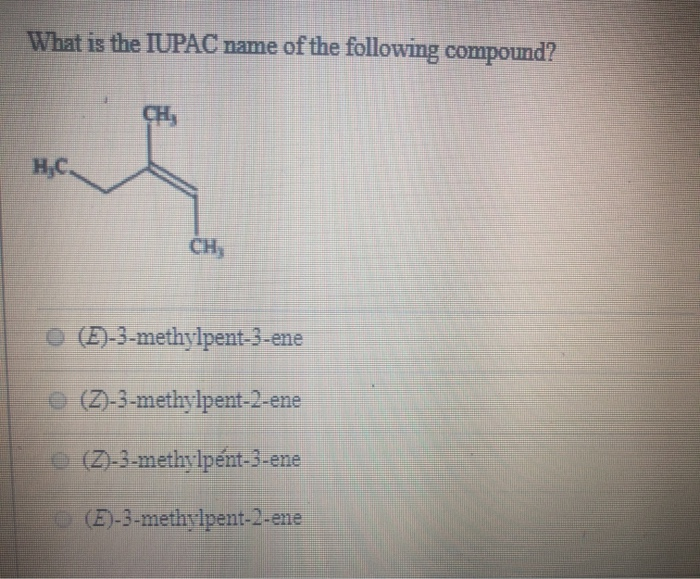



What Is The Iupac Name Of The Following Compound Eh Chegg Com



E 3 Methylpent 2 Ene Molbase




Z 3 Methylpent 2 En 1 Ol Structure C6h12o Over 100 Million Chemical Compounds Mol Instincts




Help Mcat



Alkenes Lecture Ppt Download




Draw The Structure S Of The Alkene S With The Molecular Formula C6h12 That Have A Single Methyl Homeworklib
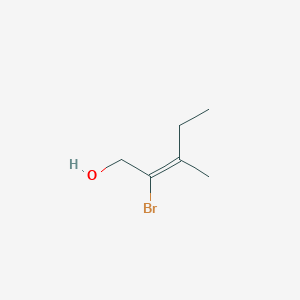



E 2 Brom 3 Methylpent 2 En 1 Ol C6h11bro Pubchem



2 Ethyl 3 Methyl 1 Pentene



Http Www Dynamicscience Com Au Tester Solutions1 Chemistry Organic Lesson 2 naming twofunctionalgroupssoln Pdf



3 Methylpent 2 Ene E 3 Methylpent 2 Ene Molcalc




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
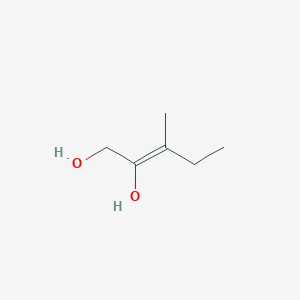



Z 3 Methylpent 2 Ene 1 2 Diol C6h12o2 Pubchem



2z 4 Methylpent 2 Ene Get Quote
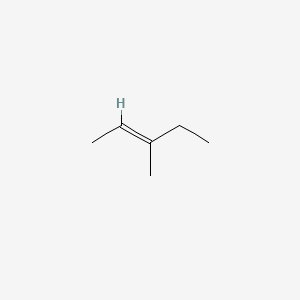



Trans 3 Methyl 2 Pentene C6h12 Pubchem



E 3 Methylpent 2 En 2 Amine Chemsink




3 Chloro 2 Methylpent 2 Ene 71 6 Wiki



E 3 Ethyl 4 Methylpent 2 Ene




13 0 Alkenes Exam Q S Flashcards Quizlet



E 3 Chloro 2 Methyl Prop 2 En 1 Ol C4h7clo Chemspider



Solved What Is The Major Product When 3 Methylpent 2 Ene Reacts With 9 n Then H2o2 Oh H2o Course Hero
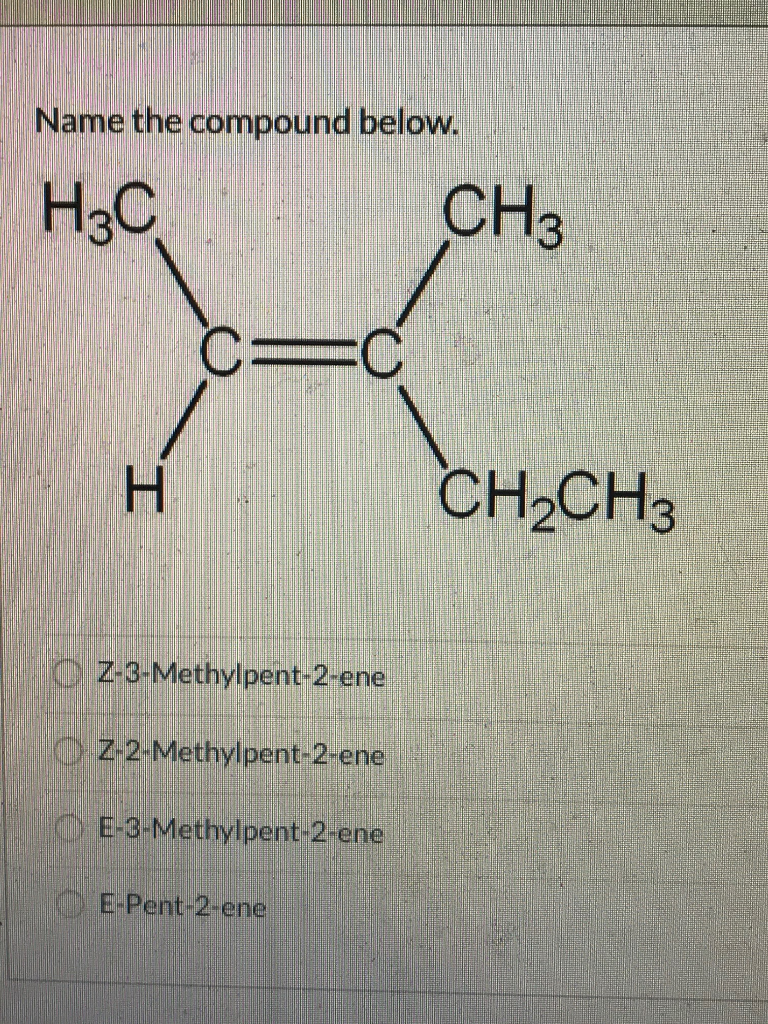



Name The Compound Below H3c Ch3 C C H Ch2ch3 Chegg Com



2 Methylpent 2 Ene Structural Formula



2 Methylpent 2 Ene C6h12 Chemspider
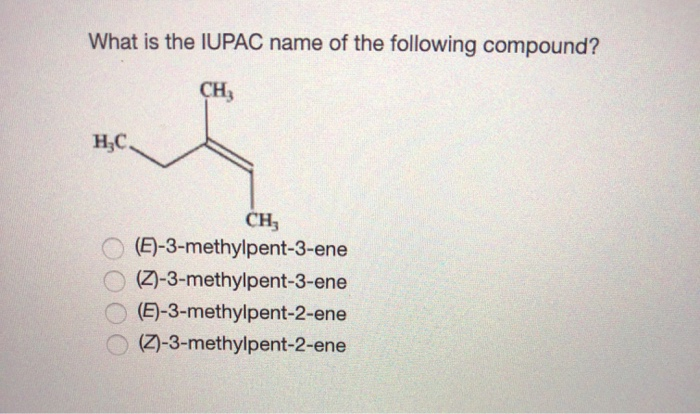



What Is The Iupac Name Of The Following Compound Ch Chegg Com



B Z 3 Methylpent 3 Ene C E 3 Methylpent 2 Ene Chegg Com
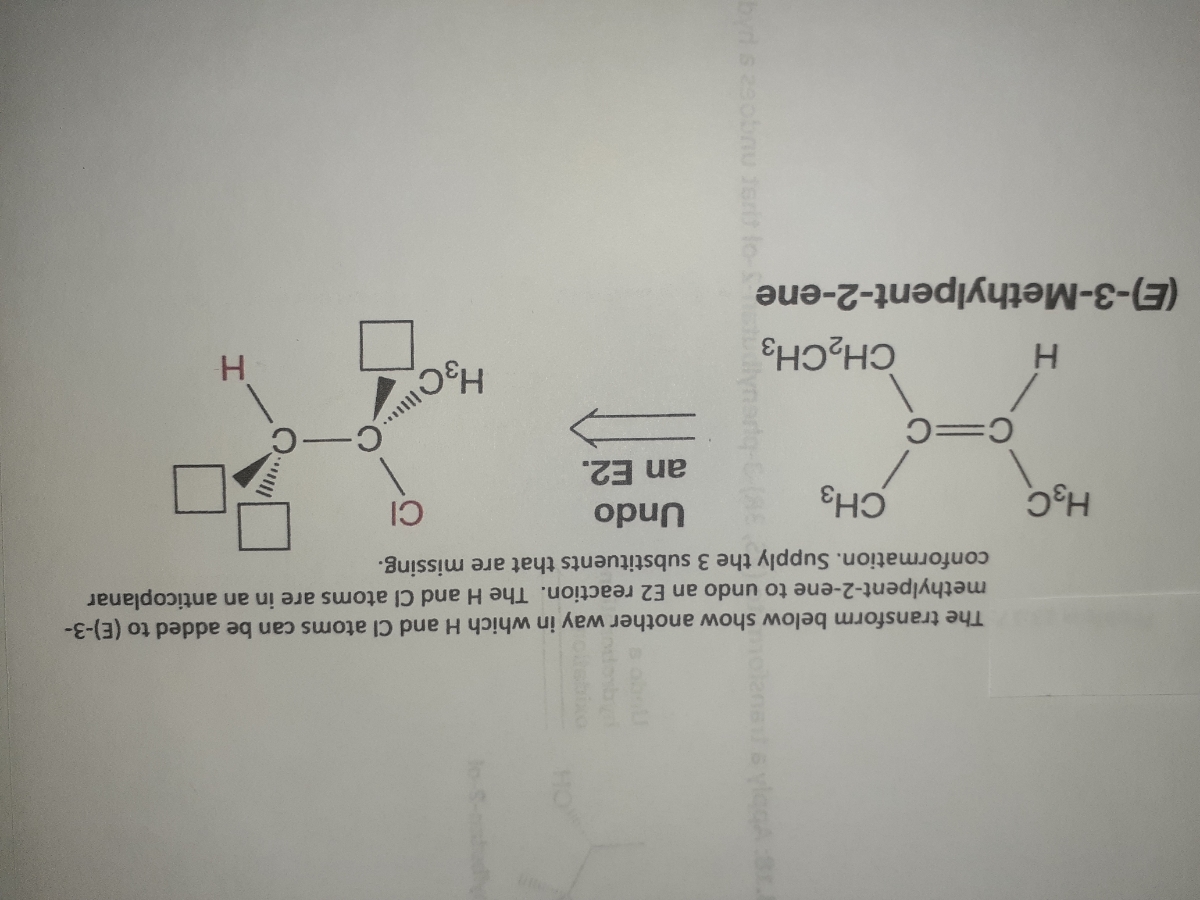



Answered The Transform Below Show Another Way In Bartleby
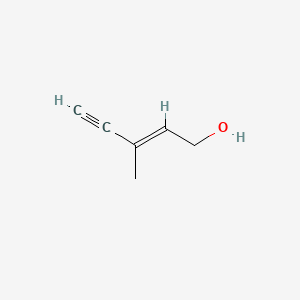



E 3 Methylpent 2 En 4 Yn 1 Ol C6h8o Pubchem




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts



Cis 3 Methylpent 2 Ene
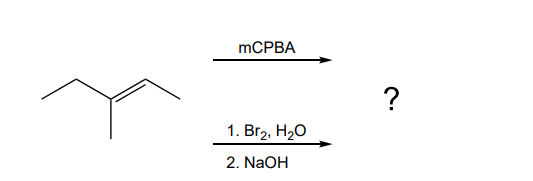



When E 3 Methylpent 2 Ene Is Reacted With Either Chegg Com
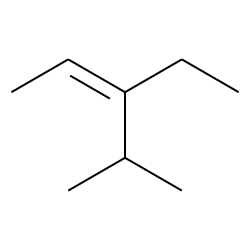



Z 3 Ethyl 4 Methylpent 2 Ene Cas 467 48 1 Chemical Physical Properties By Chemeo




3 Methylpent 2 Ene 1 5 Diol Chemical Physical Properties By Chemeo




Alfa Aesar 3 Methyl 3 Penten 2 One E Z 95 Fisher Scientific
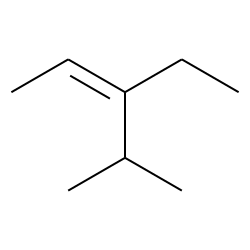



E 3 Ethyl 4 Methylpent 2 Ene Cas 467 49 2 Chemical Physical Properties By Chemeo



Echa Europa Eu Substance Information Substanceinfo 100 009 515



2e 3 Methylpent 2 Ene Get Quote




Chemsheets As006 Electron Arrangement Ppt Video Online Download




3 Methylpent 2 Ene Sigma Aldrich
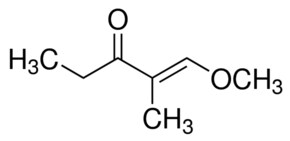



1e 1 Methoxy 2 Methyl 1 Penten 3 One 35 7




E 2 Ethoxy 3 Methylpent 2 Ene Structure C8h16o Over 100 Million Chemical Compounds Mol Instincts




Organic Chemistry Alkenes



E 3 Methylpent 2 Ene Molbase




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons



0 件のコメント:
コメントを投稿